EFFICACY
SIMULTANEOUS RELIEF of both abdominal pain & diarrhea at the same time
For the treatment of Irritable Bowel Syndrome with Diarrhea (IBS-D) in adults, the rate of composite response was numerically greater with VIBERZI than with placebo at 3 and 6 months. VIBERZI responders had dual-symptom relief.
Patients had simultaneous improvement in both abdominal pain and diarrhea as seen in two Phase 3 clinical trials1,2
RESPONDERS AT 3 and 6 months1
IBS-3002
IBS-3002 (N=1145) measured VIBERZI 100 mg (recommended dose) (n=382) vs placebo (n=382). Primary endpoint: Weeks 1-12: Trt Diff 13%, 95% CI (8%, 19%); P<0.001
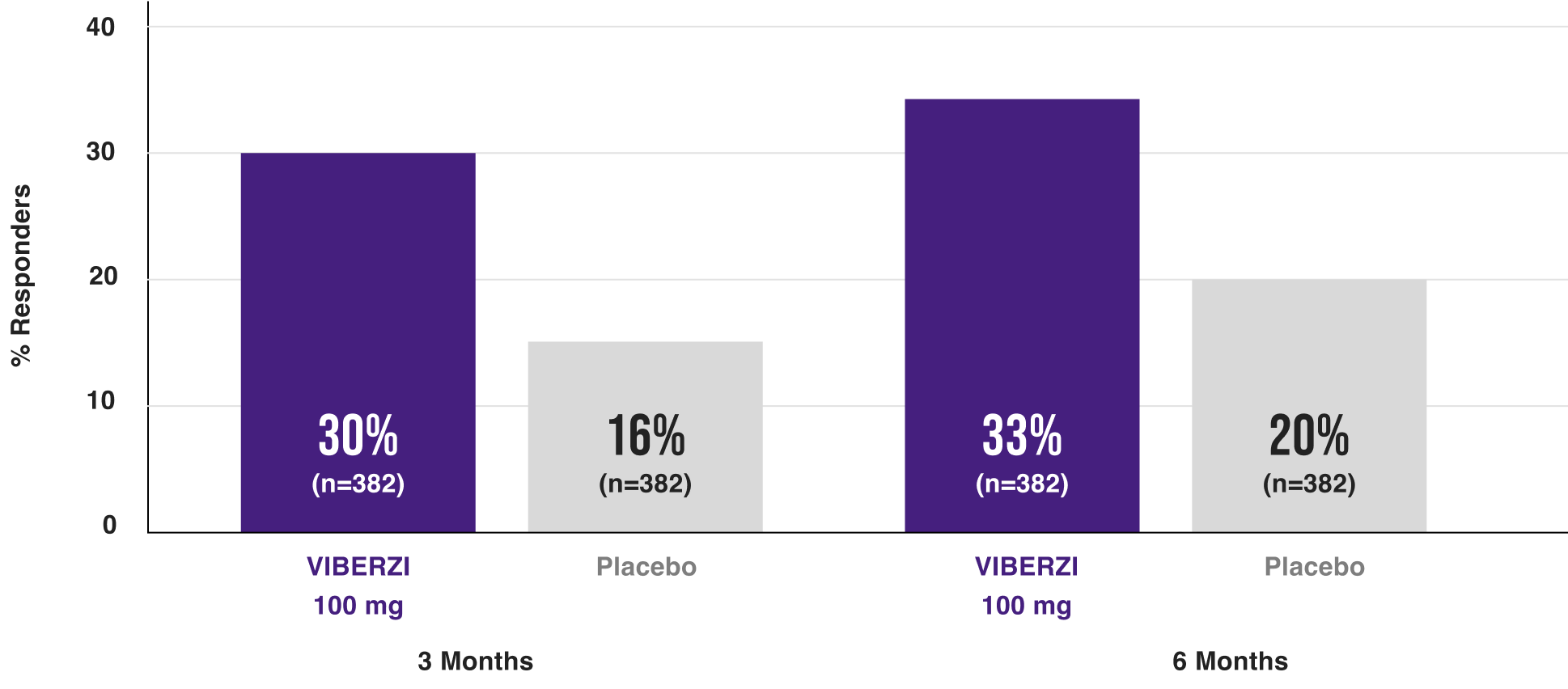
In study IBS-3001,1 Significantly more viberzi 100 mg-treated patients were responders vs placebo AT 3 MONTHS
Primary endpoint: Weeks 1-12: 25% vs 17% with placebo | Secondary endpoint: Weeks 1-26: 29% vs 19% with placebo
LASTING RELIEF of both abdominal pain & diarrhea for up to 6 months
VIBERZI provided lasting relief of both abdominal pain and diarrhea in IBS-D.
Relief of both symptoms was maintained throughout treatment1,2
In clinical trials, the proportion of patients who were combined responders to VIBERZI at each 4-week interval was numerically higher than placebo as early as Month 1 through Month 61
RESPONDERS THROUGHOUT TREATMENT INTERVALS2
IBS-3002
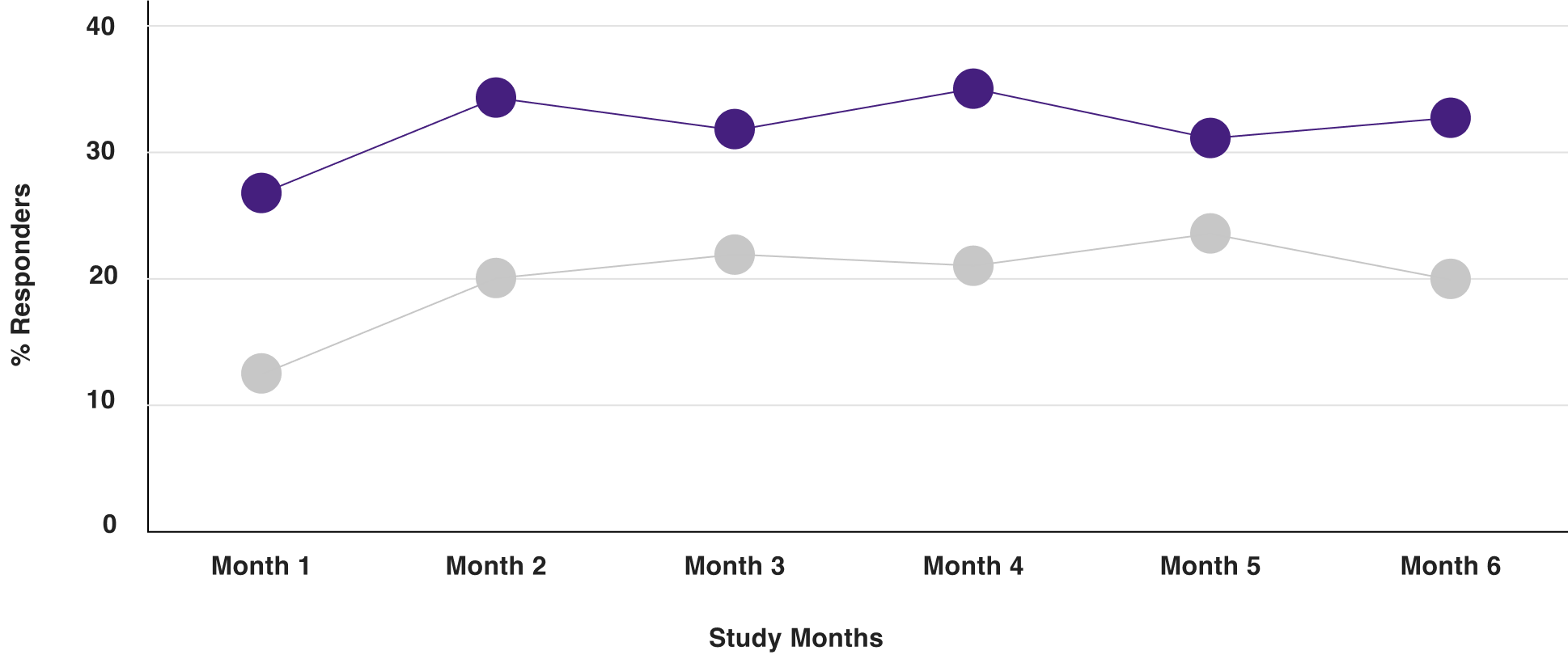
Data from a randomized, parallel-group, multicenter, multinational, double-blind, placebo-controlled, Phase 3 clinical trial in IBS-D adult patients aged 18-80 years. Graphs depict response rate for study population at each monthly interval (additional analyses).1,2
PROACTIVE RELIEF that HELPS keep IBS-D symptoms under control and helps patients get ahead of their symptoms
VIBERZI, taken twice daily, was associated with reduced symptoms of IBS-D at 6 months.1,2
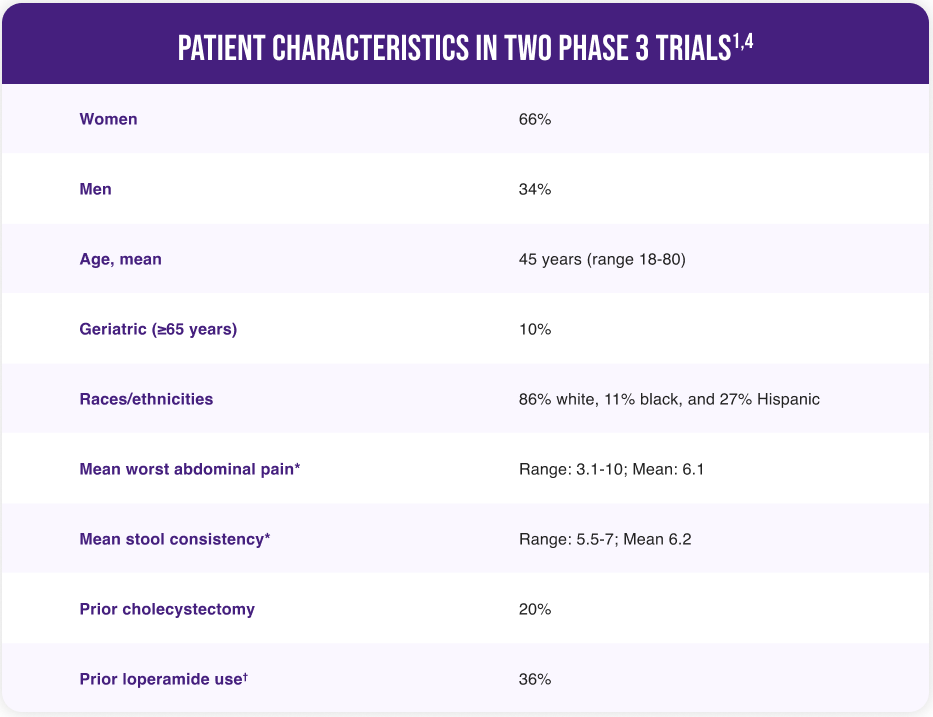
VIBERZI WAS EVALUATED IN OVER 2400 DIAGNOSED IBS-D PATIENTS
All patients met Rome III criteria for IBS-D (ie, a history of recurrent abdominal pain accompanied by frequent loose or watery stools)1,3
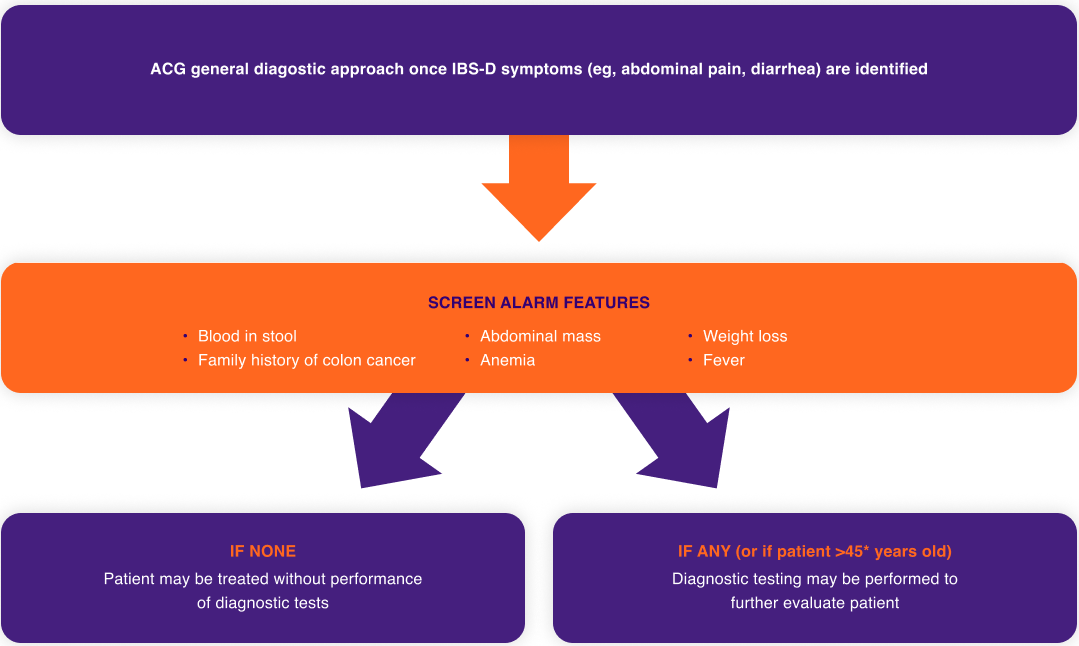
DIAGNOSTIC CRITERIA* FOR IRRITABLE BOWEL SYNDROME (IBS)3
Rome III
Recurrent abdominal pain or discomfort† on at least 3 days per month in the last 3 months associated with 2 or more of the following:
- Improvement with defecation
- Onset associated with a change in frequency of stool
- Onset associated with change in form (appearance) of stool
Subtype identification criteria for IBS with diarrhea (IBS-D)3‡:
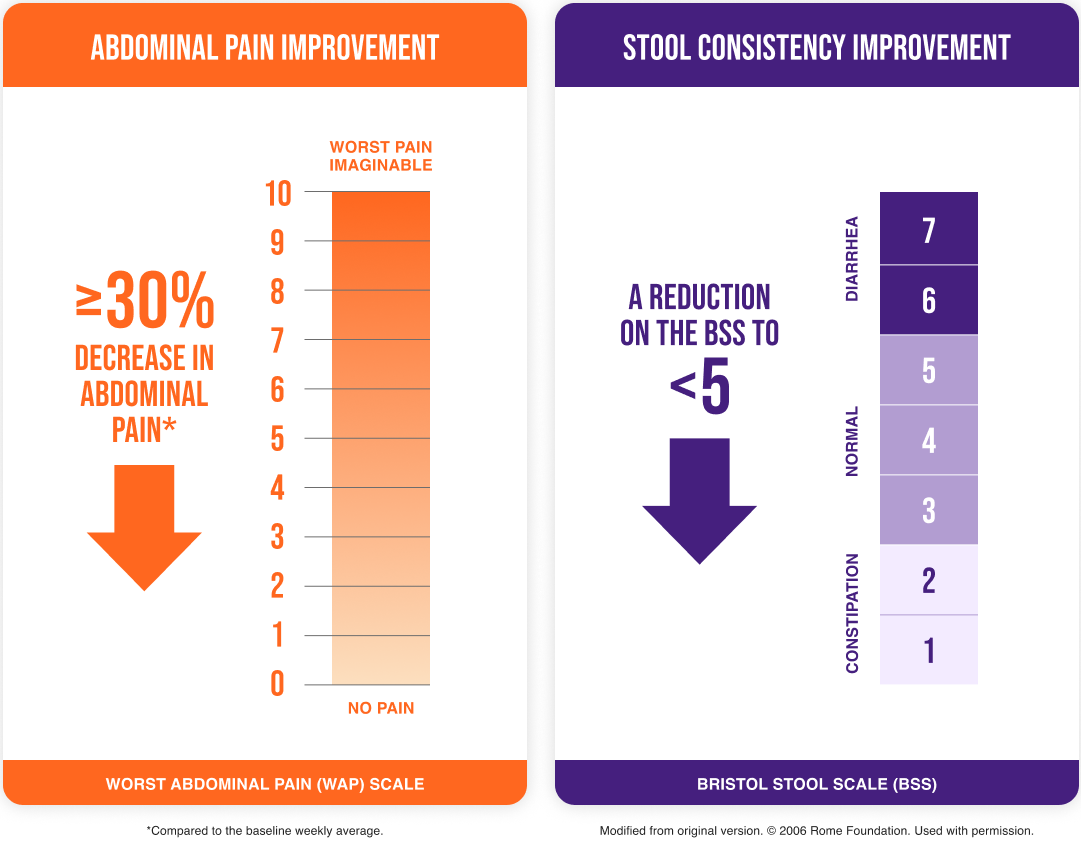
- ≥25% loose (mushy) or watery stools (a score of 6 or 7 on the Bristol Stool Scale [BSS])
- ≥25% hard or lumpy stools (a score of 1 or 2 on the BSS)
Baseline inclusion criteria1§:
- An average Worst Abdominal Pain (WAP) score of >3.0 in the past 24 hours
- An average daily Bristol Stool Scale (BSS) score of ≥5.5 and at least 5 days with a BSS score of ≥5

VIBERZI SAMPLES
Free samples available for your patients!
ORDER HERE >Not available where prohibited by law.
VIBERZI® (eluxadoline) CIV is indicated in adults for the treatment of irritable bowel syndrome with diarrhea (IBS-D).
VIBERZI is contraindicated in patients:
- Without a gallbladder.
- With known or suspected biliary duct obstruction, or sphincter of Oddi disease or dysfunction; a history of pancreatitis; or structural diseases of the pancreas.
- With alcoholism, alcohol abuse, alcohol addiction, or who drink more than 3 alcoholic beverages per day.
- With a known hypersensitivity reaction to VIBERZI.
- With severe hepatic impairment.
- With a history of chronic or severe constipation or sequelae from constipation, or known or suspected mechanical gastrointestinal obstruction.
- Pancreatitis, with or without sphincter of Oddi spasm, has been reported in patients taking either the 75 mg or 100 mg dosage of VIBERZI, including serious cases resulting in hospitalization, primarily in patients without a gallbladder. Fatal cases have also been reported in patients without a gallbladder. VIBERZI is contraindicated in patients without a gallbladder. Most of the reported cases of serious pancreatitis occurred within a week of starting treatment with VIBERZI and some patients developed symptoms after one to two doses.
- In patients with a gallbladder, evaluate a patient’s alcohol intake prior to starting VIBERZI. Instruct patients to avoid chronic or acute excessive alcohol use while taking VIBERZI. Monitor for new or worsening abdominal pain that may radiate to the back or shoulder, with or without nausea and vomiting. Instruct patients to immediately stop VIBERZI and seek medical attention if they experience symptoms suggestive of pancreatitis such as acute abdominal or epigastric pain radiating to the back or shoulder associated with elevations of pancreatic enzymes with or without nausea and vomiting.
- There is a risk of sphincter of Oddi spasm, resulting in pancreatitis or hepatic enzyme elevation associated with acute abdominal pain (eg, biliary-type pain) in patients taking VIBERZI. Serious adverse reactions of sphincter of Oddi spasm with or without pancreatitis resulting in hospitalization have been reported, primarily in patients without a gallbladder. Cases of serious sphincter of Oddi spasm occurred within a week of starting treatment with VIBERZI and some patients developed symptoms after one to two doses.
- Instruct patients to immediately stop VIBERZI and seek medical attention if they experience symptoms suggestive of sphincter of Oddi spasm such as acute worsening of abdominal pain that may radiate to the back or shoulder with or without nausea and vomiting, associated with elevations of pancreatic enzymes or liver transaminases. Do not restart VIBERZI in patients who developed biliary duct obstruction while taking VIBERZI.
- In postmarketing experience, serious hypersensitivity reactions (including anaphylaxis) have been reported following VIBERZI administration. Some of these reactions occurred after the first one or two doses of VIBERZI.
- Instruct patients to immediately stop VIBERZI and seek medical attention if they experience symptoms suggestive of a hypersensitivity reaction.
- Constipation, sometimes requiring hospitalization, has been reported following VIBERZI administration. In postmarketing experience, severe cases with development of intestinal obstruction, intestinal perforation, and fecal impaction, requiring intervention, have also been reported. Instruct patients to stop VIBERZI and immediately contact their healthcare provider if they experience severe constipation. Avoid use with other drugs that may cause constipation.
The most commonly reported adverse reactions (incidence >5% and greater than placebo) were constipation, nausea, and abdominal pain.
Please also see full Prescribing Information.