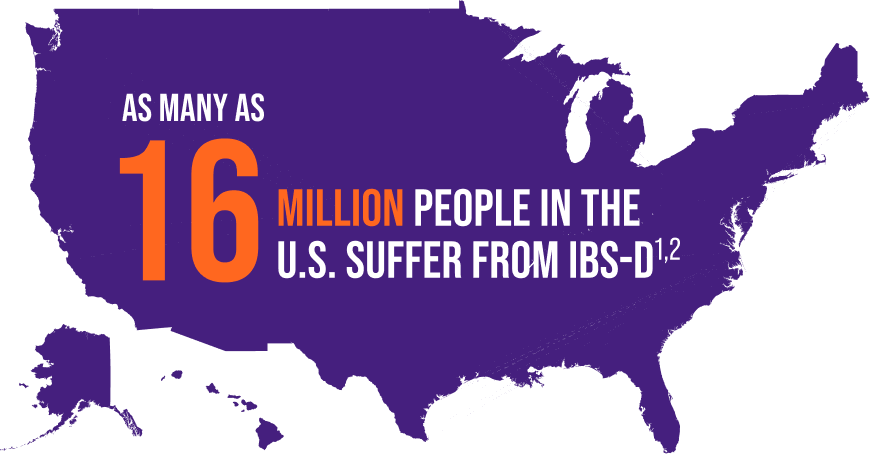
ABOUT IBS-D

VIBERZI SAMPLES
Free samples available for your patients!
ORDER HERE >Not available where prohibited by law.
VIBERZI® (eluxadoline) CIV is indicated in adults for the treatment of irritable bowel syndrome with diarrhea (IBS-D).
VIBERZI is contraindicated in patients:
- Without a gallbladder.
- With known or suspected biliary duct obstruction, or sphincter of Oddi disease or dysfunction; a history of pancreatitis; or structural diseases of the pancreas.
- With alcoholism, alcohol abuse, alcohol addiction, or who drink more than 3 alcoholic beverages per day.
- With a known hypersensitivity reaction to VIBERZI.
- With severe hepatic impairment.
- With a history of chronic or severe constipation or sequelae from constipation, or known or suspected mechanical gastrointestinal obstruction.
- Pancreatitis, with or without sphincter of Oddi spasm, has been reported in patients taking either the 75 mg or 100 mg dosage of VIBERZI, including serious cases resulting in hospitalization, primarily in patients without a gallbladder. Fatal cases have also been reported in patients without a gallbladder. VIBERZI is contraindicated in patients without a gallbladder. Most of the reported cases of serious pancreatitis occurred within a week of starting treatment with VIBERZI and some patients developed symptoms after one to two doses.
- In patients with a gallbladder, evaluate a patient’s alcohol intake prior to starting VIBERZI. Instruct patients to avoid chronic or acute excessive alcohol use while taking VIBERZI. Monitor for new or worsening abdominal pain that may radiate to the back or shoulder, with or without nausea and vomiting. Instruct patients to immediately stop VIBERZI and seek medical attention if they experience symptoms suggestive of pancreatitis such as acute abdominal or epigastric pain radiating to the back or shoulder associated with elevations of pancreatic enzymes with or without nausea and vomiting.
- There is a risk of sphincter of Oddi spasm, resulting in pancreatitis or hepatic enzyme elevation associated with acute abdominal pain (eg, biliary-type pain) in patients taking VIBERZI. Serious adverse reactions of sphincter of Oddi spasm with or without pancreatitis resulting in hospitalization have been reported, primarily in patients without a gallbladder. Cases of serious sphincter of Oddi spasm occurred within a week of starting treatment with VIBERZI and some patients developed symptoms after one to two doses.
- Instruct patients to immediately stop VIBERZI and seek medical attention if they experience symptoms suggestive of sphincter of Oddi spasm such as acute worsening of abdominal pain that may radiate to the back or shoulder with or without nausea and vomiting, associated with elevations of pancreatic enzymes or liver transaminases. Do not restart VIBERZI in patients who developed biliary duct obstruction while taking VIBERZI.
- In postmarketing experience, serious hypersensitivity reactions (including anaphylaxis) have been reported following VIBERZI administration. Some of these reactions occurred after the first one or two doses of VIBERZI.
- Instruct patients to immediately stop VIBERZI and seek medical attention if they experience symptoms suggestive of a hypersensitivity reaction.
- Constipation, sometimes requiring hospitalization, has been reported following VIBERZI administration. In postmarketing experience, severe cases with development of intestinal obstruction, intestinal perforation, and fecal impaction, requiring intervention, have also been reported. Instruct patients to stop VIBERZI and immediately contact their healthcare provider if they experience severe constipation. Avoid use with other drugs that may cause constipation.
The most commonly reported adverse reactions (incidence >5% and greater than placebo) were constipation, nausea, and abdominal pain.
Please also see full Prescribing Information.